Project
Background
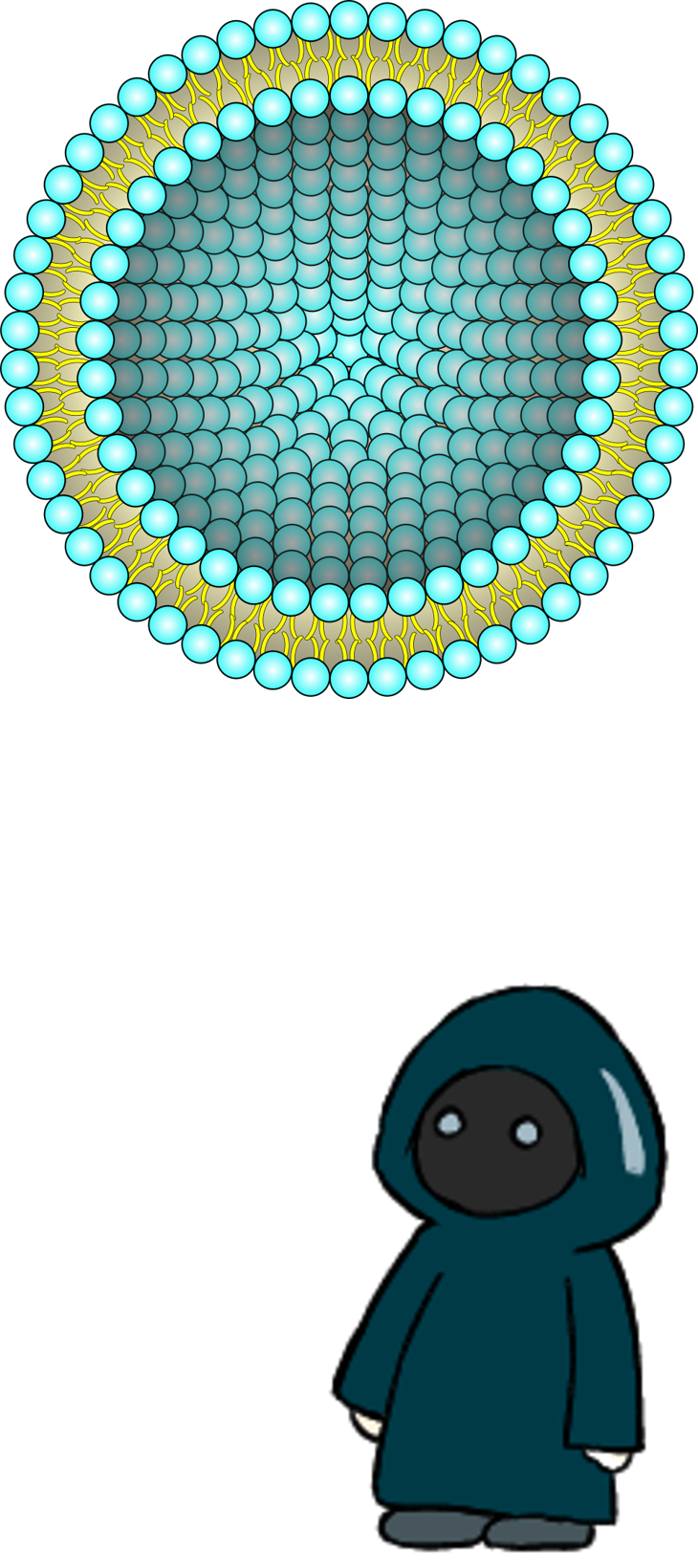
〇About liposome
Liposomes have lipid bilayer membranes composed of phospholipids. Phospholipid molecules are amphipathic structures linked with a hydrophilic head and a hydrophobic tail. This property allows phospholipids to be self-assembled to form spherical liposomes[1]. Modifying various biomolecules on the membrane surface and encapsulating biomolecules inside a liposome can add various liposome functions. One of the typical applications is drug delivery systems (DDS) using liposomes as carriers. Many kinds of liposomes such as pH-sensitive[2] or temperature-sensitive liposomes[3] in vivo environments have been established. Ligand-modified liposomes, such as hapten liposomes that do not induce immunogenicity and stealth liposomes, are also used[4] as a means of DDS by encapsulating drugs by thin film hydration methods[5], emulsification methods[5], etc. Another promising application of liposomes is playing compartment roles in molecular systems and robots composed of designed biomolecules[6][7][8]. Practical examples include the control of motor protein kinesin behavior within liposomes and the amplification of single-stranded DNA inside liposomes[9].
〇About DNA nanotechnology
Molecular robotics utilize DNA nanotechnology in addition to liposome technology. DNA nanotechnology is a research field that constructs nanometer-sized structures by self-assembling sequence-designed DNA developed by Ned Seeman[10][11]. DNA contains information, determined by the order of the sequences, while it also has the property of forming structures by designing its sequence. Nucleotides (4 types) that compose DNA are significantly fewer than amino acids (20 types) that form proteins, which makes DNA easier to design and control.
Furthermore, various molecules can be modified into DNA strands, such as fluorescent molecules and cholesterol to the DNA strand, allowing the addition or modulation of physical and chemical properties. In addition, since DNA is a biocompatible biomolecule, that can be applied in a wide range of fields both in vitro and in vivo. Typical examples of sequence-designed DNA structures are DNA origami[12] and DNA hydrogel[13], which are described below.
〇About DNA hydrogel
Hydrogels are materials that form three-dimensional networks physically or chemically crosslinked by soluble or hydrophilic polymers They are expected to be applied in various fields such as DDS carriers,[14] sensors[15], and biological tissues in medical applications[16]. In the last several decades, the modification of DNA strands into hydrogels has enabled the construction of DNA hydrogels that combine the properties and advantages of both hydrogels and DNA origamis[17][18]. For example, DNA hydrogels have been investigated to control the ability to change swelling, cross-link density, and optical or mechanical properties in response to external stimuli[19], and to protect drugs[20].
〇About DNA origami
DNA origami is a technology that can construct two[12] or three-dimensional[21][22] nanostructures with arbital shapes by self-assembling a circular scaffold DNA strand with several thousands of bases with ~ 200 types of short DNA strands[23]. The property of DNA complementary and hybridization allows to build a complex structure with nanometer order accuracy. Since DNA and DNA origami nanostructures are hydrophilic molecules, they cannot interact with hydrophobic molecules, lipid molecules, and lipid membranes. However, by using cholesterol modifications and charge properties, DNA origami nanostructures can add various functions to the liposome membrane. Examples of implementing DNA origami structures to liposome membranes include membrane deformation by structure accumulation[24][25][26] and forming nanopores to the membrane surface[27][28][29]. The deformation of the membrane surface is achieved by the gathering of cholesterol-modified DNA nanostructures on the membranes of the liposomes. The lipid membrane of liposomes separates the internal and external environments, not allowing the molecular exchange between inner and outer environments. This molecular exchange can be conducted by constructing pores that serve molecular transportation pathways using DNA origami., The binding and penetration into the lipid membrane of DNA origami nanopore is induced by dozens of cholesterol modifications.
Problem1
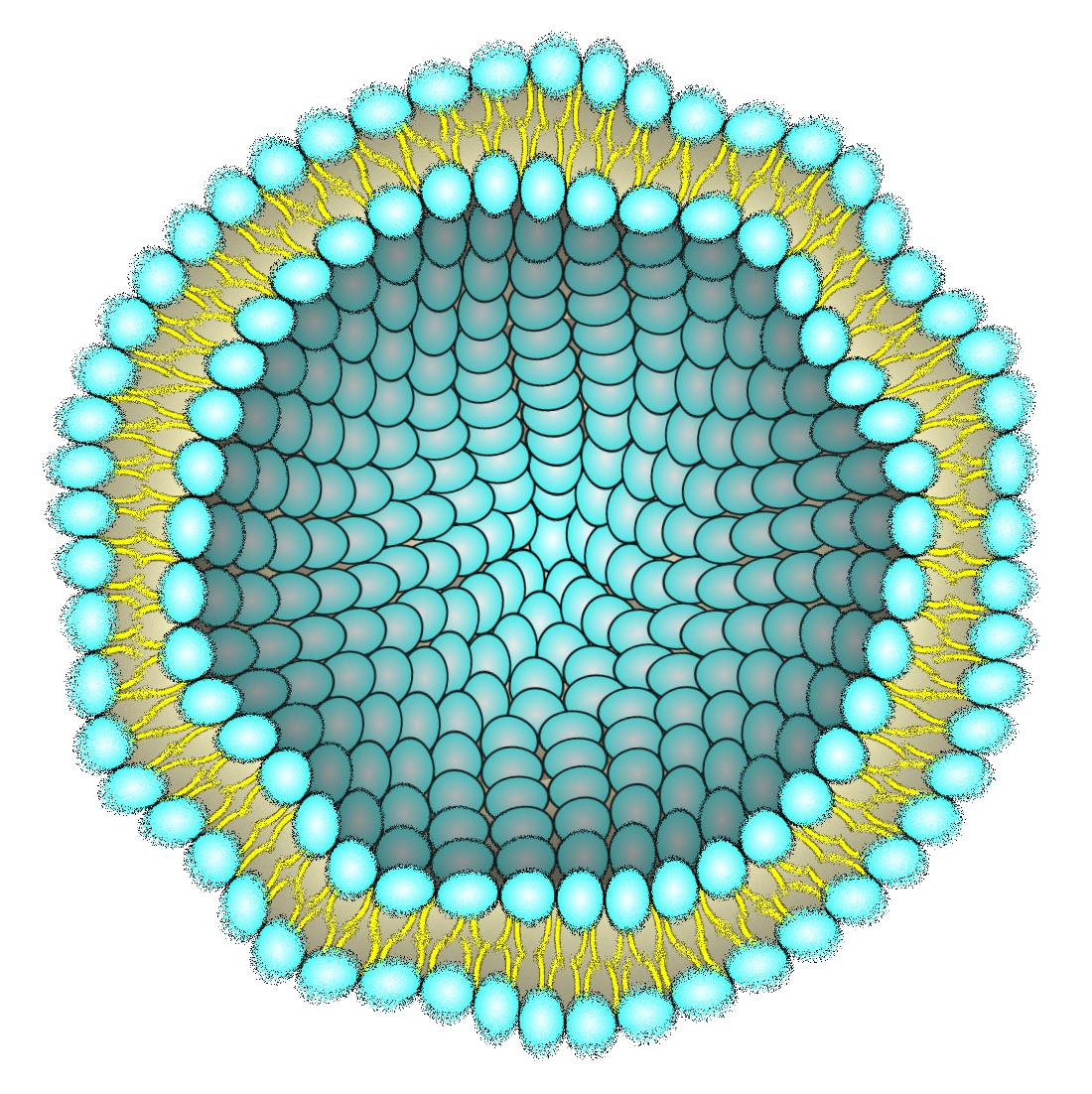
Liposomes are used in vitro, in vivo, and in various natural environments. However, changes in the external environment, such as osmotic pressure[30], and temperature at which the lipid bilayer undergoes a phase transition[31], or external forces affect the stability of liposomes. These cause concerns about leaking of the inner components and this becomes a barrier to further applications. To overcome these concerns, the research for increasing the stability of liposomes keeps going. Previous studies have attempted to stabilize liposomes by preparing a DNA cytoskeleton inside the lipid bilayer[32][33][34][35] or by modifying polyethylene glycols (PEG) [4][36][37] or cholesterol[38][39] on the liposome membrane surface. Moreover, Maruyama et al. have demonstrated that modifying the liposome membrane surface with PEG significantly increases the blood circulation half-life compared to liposomes without such modifications[4].
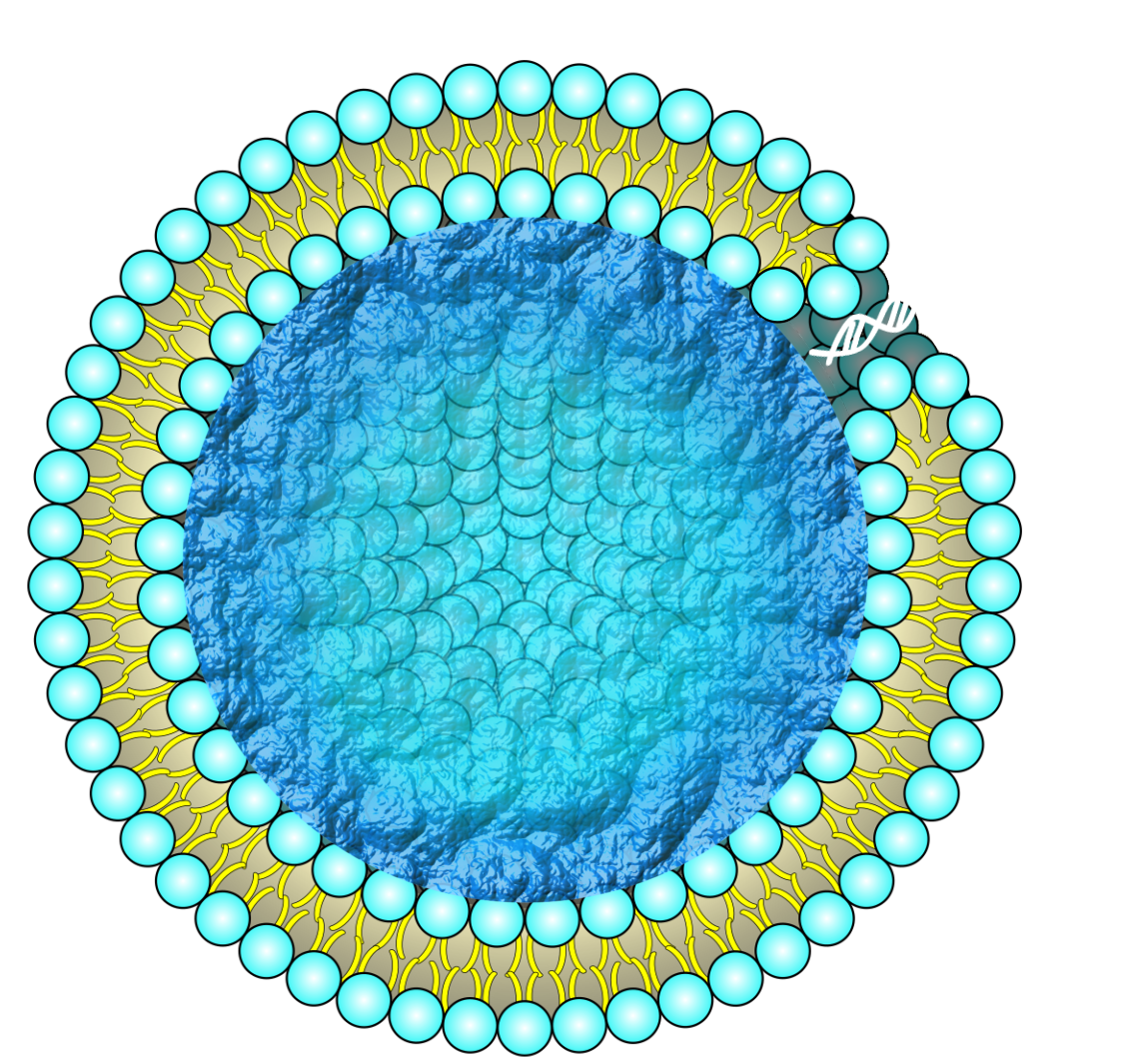
Problem2
However, such tools for stabilizing liposomes might become obstacles in implementing molecular transportation systems in the future. DNA hydrogel and PEG modification are utilized to improve the stability of liposomes. Exchanging molecules and signals with external environments is essential to expand the function of artificial cells and molecular robots. In previous research, the molecular transporting via liposome membranes is realized by embedding membrane proteins such as a-hemolysin [40], Streptolysin O[41], and nanopore-forming DNA nanostructures in liposome membranes[42]. As far as we know, these nanopores aimed to form pores to conventional Giant unilamellar vesicles (GUV) and it is unclear that these nanopores can form pores to GUVs with DNA hydrogel or PEG modifications. Since these nanopore devices cannot penetrate natural cell membranes[9][43], which have a complicated composition, it is similarly difficult to form pores toward GUV-coated DNA hydrogel. So, a new method of forming pore devices and systems is essential to work together to improve stability and molecular transformation systems.

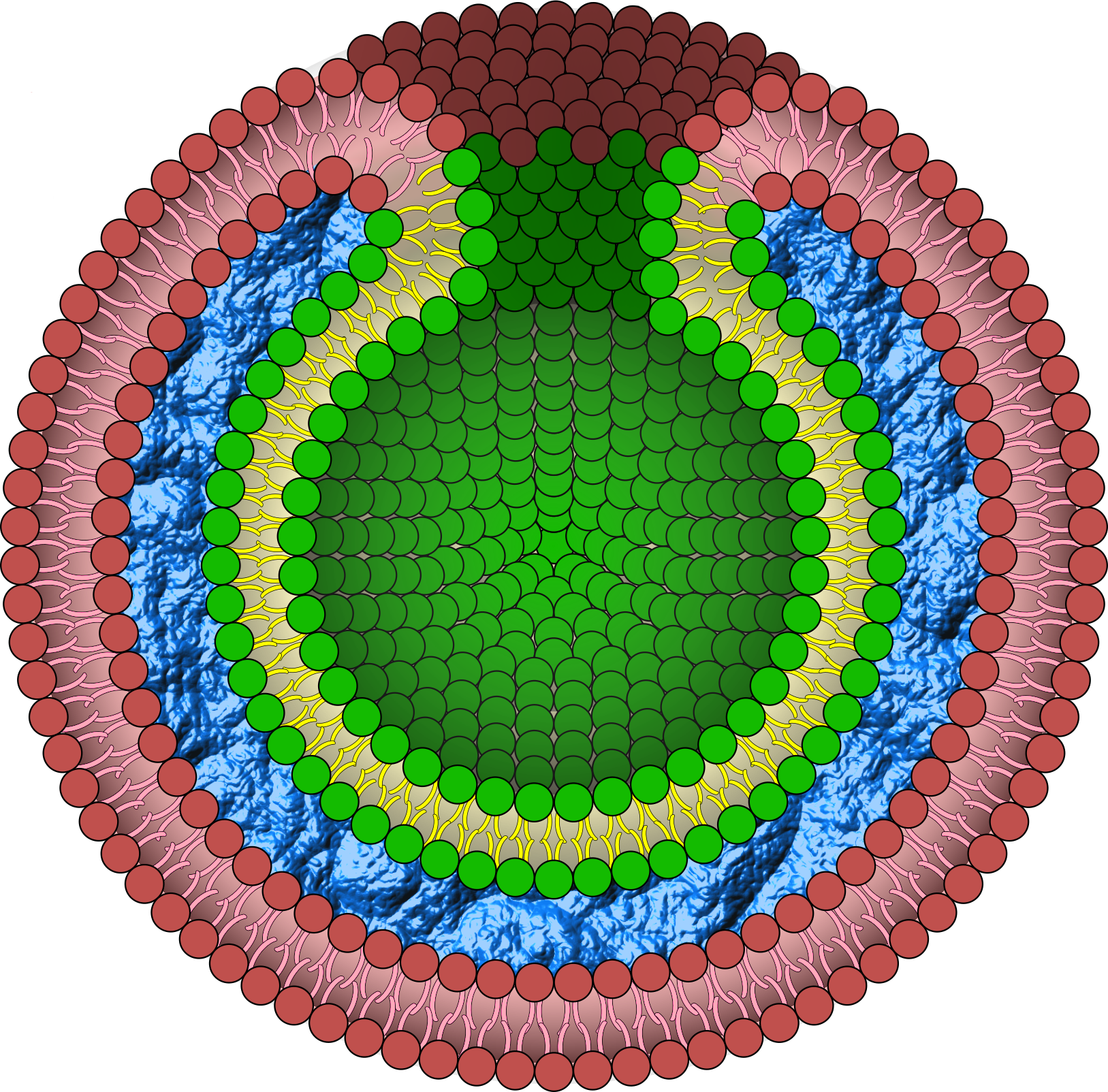
Solution
In this project, we propose a nuclear envelope-like structure that is both robust and capable of future molecular transport through lipid membranes. We designed a triple-layer capsule structure, BGB (Bilayer-Gel-Bilayer), in which the DNA network is packed into two lipid bilayer layers. Different from previous studies, liposome membranes were used to sandwich both sides of the DNA network. We expected that the DNA network could increase the stability of the capsule structure without exposure and affect the inner component[32]. We also applied a gimmick that brings the inner bilayer and outer bilayer into contact. DNA-origami curls attached to the inner bilayer automatically polymerize, resulting in the formation of a tube from the inner bilayer. This mechanism creates pores in the BGB structure, enabling molecular transport between the inside and outside of the liposome membrane.BGB structure on our project will establish a new method of DDS and compartment mimicking living cell as artificial cell models.
Goals
Reference
- Abolfazl Akbarzadeh, Rogaie Rezaei-Sadabady, Soodabeh Davaran, Sang Woo Joo, Nosratollah Zarghami, Younes Hanifehpour, Mohammad Samiei, Mohammad Kouhi & Kazem Nejati-Koshki, Nanoscale Research Letters, 2013, Volume 8, article number 102.
- Haitao Ding, Ping Tan, Shiqin Fu, Xiaohe Tian, Hu Zhang, Xuelei Ma, Zhongwei Gu, Kui Luo, Journal of Controlled Release, 2022, Volume 348, 206-238.
- Waad H. Abuwatfa, Nahid S. Awad, William G. Pitt and Ghaleb A. Husseini, Polymers, 2022, Volume 14, Issue 5, 925.
- Alexander L. Klibanov, Kazuo Maruyama, Vladimir P. Torchilin, Leaf Huang, FEBS Letters, 1990, Volume 268, Issue 1, 235-237.
- Luka Šturm and Nataša Poklar Ulrih, IJMS, 2021, Volume 22 Issue 12, 6547.
- Satoshi Murata, Akihiko Konagaya, Satoshi Kobayashi, Hirohide Saito & Masami Hagiya, New Generation Computing, 2013, Volume 31, 27–45.
- Kan Shoji and Ryuji Kawano, Micromachimes, 2020, Volume 11 Issue 9, 788.
- Zugui Peng, Shoji Iwabuchi, Kayano Izumi, Sotaro Takiguchi, Misa Yamaji, Shoko Fujita, Harune Suzuki, Fumika Kambara, Genki Fukasawa, Aileen Cooney, Lorenzo Di Michele, Yuval Elani, Tomoaki Matsuura and Ryuji Kawano, Lab on a Chio, 2024, Issue 5, 996-1029.
- Zhaowei Chen, Jinqiang Wang, Wujin Sun, Edikan Archibong, Anna R Kahkoska, Xudong Zhang, Yue Lu, Frances S Ligler, John B Buse & Zhen Gu, Nature Chemical Biology, 2018, 14, 86-93.
- Nadrian C. Seeman, Journal of Theoretical Biology, 1982, Volume 99, Issue 2, 237-247.
- Nadrian C. Seeman and Hanadi F. Sleiman, Nature Reviews Materials, 2018, 3, Article number: 17068.
- Erik Winfree, Furong Liu, Lisa A. Wenzler and Nadrian C. Seeman, Nature, 1998, 394, 539-544.
- Dr. Huiling Jiang, Victor Pan, Skanda Vivek, Prof. Eric R. Weeks and Prof. Yonggang Ke, ChemBioChem, 2016, Volume 17, Issue 12, 1156-1162.
- Heping Qiu, Hui Guo, Di Li, Yuchuan Hou, Tairong Kuang, Jianxun Ding, Trends in Biotechnology, 2020, Volume 38, Issue 6, 579-583.
- Chao Zhang, Yongsen Zhou, Haijie Han, Huanxi Zheng, Wanghuai Xu and Zuankai Wang, ACS Nano, 2021, 15, 1, 1785–1794.
- Xinyue Liu, Christoph Steiger, Shaoting Lin, German Alberto Parada, Ji Liu, Hon Fai Chan, Hyunwoo Yuk, Nhi V. Phan, Joy Collins, Siddartha Tamang, Giovanni Traverso and Xuanhe Zhao, Nature Communications volume, 2019, 10, Article number: 493.
- Soong Ho Um, Jong Bum Lee, Nokyoung Park, Sang Yeon Kwon, Christopher C. Umbach and Dan Luo, Nature Materials volume, 2006, 5, 797–801.
- Vinod Morya, Shanka Walia, Biman B Mandal, Chinmay Ghoroi and Dhiraj Bhatia, ACS Biomater. Sci. Eng., 2020, 6, 11, 6021–6035.
- Fengyun Li, Danya Lyu, Shuo Liu and Weiwei Guo, Advanced Materials, 2019, Volume 32, Issue 3, 1806538.
- Todd R. Hoare and Daniel S. Kohane, Polymer, 2008, Volume 49, Issue 8, 1993-2007.
- Ebbe S. Andersen, Mingdong Dong, Morten M. Nielsen, Kasper Jahn, Ramesh Subramani, Wael Mamdouh, Monika M. Golas, Bjoern Sander, Holger Stark, Cristiano L. P. Oliveira, Jan Skov Pedersen, Victoria Birkedal, Flemming Besenbacher, Kurt V. Gothelf and Jørgen Kjems,Nature, 2009, 459, 73-76.
- Shawn M. Douglas, Hendrik Dietz, Tim Liedl, Björn Högberg, Franziska Graf and William M. Shih, Nature, 2009, 459, 414-418.
- Paul W. K. Rothemund, Nature, 2006, 440, 297-302.
- Dr. Aleksander Czogalla, Dominik J. Kauert, Dr. Henri G. Franquelim, Dr. Veselina Uzunova, Dr. Yixin Zhang, Prof. Ralf Seidel, Prof and Petra Schwille, Angewandte Chemie International Edition, 2015, Volume 54, Issue 22, 6501-6505.
- Michael W. Grome, Dr. Zhao Zhang, Dr. Frédéric Pincet and Prof. Chenxiang Lin, Angewandte Chemie International Edition, 2018, Volume 57, Issue 19, 5330-5334..
- Henri G. Franquelim, Hendrik Dietz and Petra Schwille, Soft Matter, 2021, 17, Issue 2, 276-287.
- Martin Langecker, Vera Arnaut, Thomas G. Martin, Jonathan List, Stephan Renner, Michael Mayer, Hendrik Dietz and Friedrich C. Simmel, Science, 2012, Vol 338, Issue 6109, 932-936.
- Shoji Iwabuchi, Ibuki Kawamata, Satoshi Murata and Shin-ichiro M. Nomura, Chem. Commun., 2021, 57, 2990-2993.
- Swarup Dey, Adam Dorey, Leeza Abraham, Yongzheng Xing, Irene Zhang, Fei Zhang, Stefan Howorka and Hao Yan, Nature Communications volume, 2022, 13, Article number: 2271.
- J. Sabın, G. Prieto, J. M. Ruso, R. Hidalgo-Álvarez & F. Sarmiento, The European Physical Journal E, 2006, Volume 20, 401-408.
- Michael Anderson and Abdelwahab Omri,Taylor & Francis, 2004, Issue 1, 33-39.
- Chikako Kurokawa, Kei Fujiwara, Masamune Morita, Ibuki Kawamata, Yui Kawagishi, Atsushi Sakai, Yoshihiro Murayama, Shin-ichiro M. Nomura, Satoshi Murata, Masahiro Takinoue, and Miho Yanagisawa, PNAS, 2017, 114(28), 7228-7233.
- Yusuke Sato and Masahiro Takinoue, JACS Au, 2022, 2, 1, 159-168.
- Kevin N. Baumann, Luca Piantanida, Javier García-Nafría, Diana Sobota, Kislon Voïtchovsky, Tuomas P. J. Knowles and Silvia Hernández-Ainsa, ACS Nano, 2020, 14, 2, 2316-2323.
- Youssef Helwa , Neeshma Dave and Juewen Liu, Soft Matter, 2013, 9, 6151-6158.
- Kayano Izumi, Jiajue Ji, Keiichiro Koiwai and Ryuji Kawano, ASC OMEGA, 2024, 9, 9, 10958-10966.
- D Papahadjopoulos, T M Allen, A Gabizon, E Mayhew, K Matthay, S K Huang, K D Lee, M C Woodle, D D Lasic and C Redemann, PNAS, 1991, 88(24), 11460-11464.
- Maria-Lucia Briuglia, Chiara Rotella, Amber McFarlane and Dimitrios A. Lamprou, Drug Delivery and Translational Research, 2015, Volume 5, 231-242.
- L Coderch, J Fonollosa, M De Pera, J Estelrich, A De La Maza, J L Parra, J Control Release, 2000, 68(1), 85-95.
- Satoshi Fujii, Tomoaki Matsuura, Takeshi Sunami, Yasuaki Kazuta and Tetsuya Yomo, PNAS, 2013, vol. 110 no. 42, 16796-16801.
- JAMES L. DUNCAN and RICHARD SCHLEGEL, The Journal Of Cell Biology, 1975, Volume 67, 160-173.
- Jonathan R. Burns, Eugen Stulz and Stefan Howorka, Nano Letters, 2013, 13, 6, 2351-2356.
- Nishkantha Arulkumaran, Conor Lanphere, Charlotte Gaupp, Jonathan R. Burns, Mervyn Singer and Stefan Howorka, ACS Nano, 2021, 15, 3, 4394-4404.